
A New Era of Autoimmunity Diagnostics in China
There are hundreds of parameters on CLIA platform ranging from autoimmunity, reproductive health, infectious diseases and routine test. We have launched unique test panels for autoimmunity such as Rheumatoid Arthritis, Connective Tissue Diseases, etc. For some rare autoimmune diseases, we also provide testing panels, and such behavior can indicate the company's principle-care for all.
In April, 2019, iModules fully automatic immunoassay integration system from Drawray's parent company YHLO, was officially installed in Department of Immunology and Rheumatology of PUMCH which will be running full panel of 6 parameters for the diagnosis ofAntiphospholipid Syndrome (APS) exclusively.
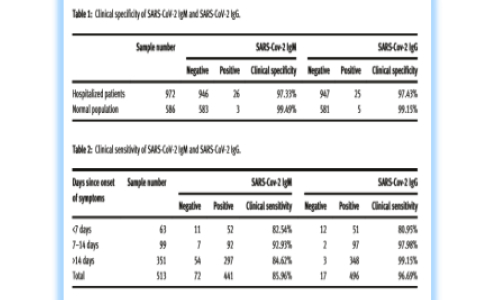
Disclose Formula for SARS-CoV-2 lgG/IgM
During the severe situation of the COVID-19, the company deeply reflected its sense of social responsibility.
We publicly test the formula for other peer companies to make medical devices and reagents against COVID-19, so as to better estimate people's health. We will spend this difficult time with the whole society. This was also world's first paper to disclose formula of SARS-CoV-2 lgGand IgM assays on CLIA platform, which helped IVD companies to launch their SARS-CoV_2 lgG and IgM assays on CLIA.

During Covid-19 Pandemic
In January 2020, just before Chinese New Year, we noticed the novel coronavirus outbreak, Drawray's parent company YHLO set up a special force team in R&D to develop SARS-CoV_2 IgG/IgM assays.With Frank Xia's experiences in 2003 SARS IgG ELISA assay, YHLO finished R&D of SARS-CoV_2 IgG/IgM on Feb 10,2020. What's more, the company had donated more than 3 millions tests of GLINE-2019-nCoVAg assays and over 1 million tests of SARS-CoV_2 IgG/lgM assays to all over the world.
Up to March 25th, 2022, the company has donated 1.2 million YHLO COVID-19Rapid Antigen Tests to Hong Kong together with Hongkong partner. On March 25th, 2022, the company and 123 people from all walks of life in Hong Kong and the Mainland China jointly initiated the establishment of the "Hong Kong Anti-epidemic Alliance".